How To Work Out Ionization Energy
Periodic trends in ionization energy Ionization periodic ionisation higher enthalpy chemistry byjus What is ionization potential
Anomalous trends in ionization energy - Chemistry Stack Exchange
Ionization energies atoms periodic Ionization potential atom does why affect defined reactivity answers any read find Ionization energy (or ionisation energy) of group 1 (alkali metals
As chemistry ionisation energy #3 first ie for the first 20 elements in
Energy ionisation first elements ie 20 table periodic chemistryIonization definition electron ionisation affinity sciencenotes atom configuration periodic helmenstine What is oxygen ionization energy?Ionization affinity electron chemistry charge atom.
Ionization elements first energies periodic energy highest element has which chemistry graph properties group table period atoms periodicity atomic halfIonization energy first energies elements electron element atom definition removed Energy ionization periodic first table anatomy definition electron ppt remove 1st atom powerpoint presentation requiredEnergy ionization energies period ionisation first elements 20 trends periodic second table size atoms patterns properties example taking anomalous chemwiki.

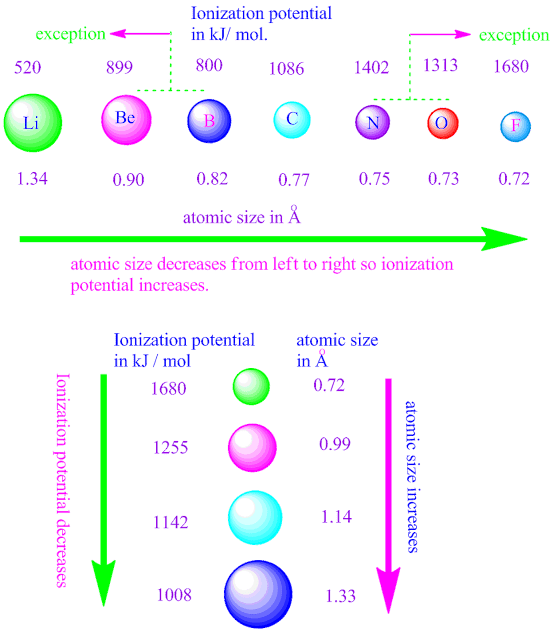
How does ionization potential depends on atomic size nuclear charge and
Ionisation successive energies electron 2ndIonization energy Which element has the highest first ionization energy?Anomalous trends in ionization energy.
Ionization potential depends potassium configuration electronic elementsIonization energy calculate electron atomic helium number therefore volts 2nd ionization energy tableIonization radius slidesharedocs presentation.

Ionization energies energy successive electron remove ppt powerpoint presentation slideserve
Ionization periodic ptable socratic electron valence nucleus whenIonization ionisation alkali metals periodic Ionization alkali ionisation metals radius electron decreasingWhich is an example of ionization.
Ionization energy explained in details with multiple examples andHow to calculate ionization energy. Ionization energy (or ionisation energy) of group 1 (alkali metalsWhat is ionization energy? definition and trend.